Biosynthesis of Folates
Tetrahydrofolate and its derivatives (folates) are essential cofactors of
one-carbon metabolism which are required for the biosyntheses of purines,
thymidylate, serine and methionine in a wide variety of organisms; they
are also required for the formylation of methionyl-tRNA in eubacteria.
Whereas plants and many microorganisms obtain folate coenzymes by de novo
synthesis, vertebrates depend on nutritional sources. Insufficient supply
of the vitamin is conducive to anemia in adults and to neural tube
malformation in human embryos.
Similar to bacteria and yeasts, plants make folates de novo from pterin,
p-aminobenzoate (PABA), and glutamate moieties. In contrast, humans and
other mammals lack a complete folate-synthesis pathway and thus need
dietary folate. Because plant foods are major folate sources, and folate
deficiency is a global health problem, enhancing plant folate content is a
prime target for metabolic engineering. This engineering demands knowledge
of the biosynthetic pathway.
The plant folate-synthesis pathway is not understood fully, but is most
probably similar to that in bacteria. The pterin
hydroxymethyldihydropteroate is formed from GTP, and PABA from chorismate.
The pterin and PABA units are condensed, glutamylated, and reduced to give
tetrahydrofolate, and a polyglutamyl tail is added.
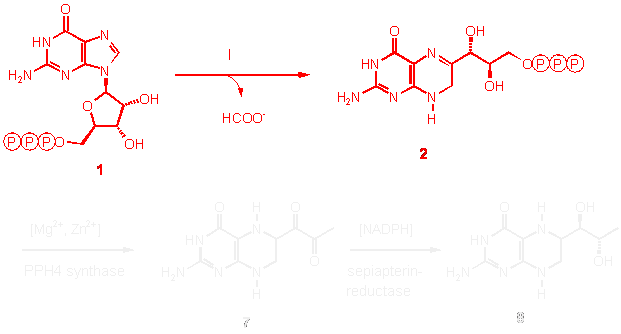
The biosynthesis of tetrahydrofolate has been studied in some detail.
The first step of pterin synthesis is of special interest, because it commits GTP (1) to pterin production and is considered to control flux into the pathway.
A pyrophosphatase (II) and a
phosphatase (III) have been proposed to convert dihydroneopterin
triphosphate to 7,8-dihydroneopterin (3) in two consecutive step, but the
details are still incompletely understood. The conversion of
7,8-dihydroneopterin into 6-hydroxymethyl-7,8-dihydroneopterin (5) and
glycolaldehyde (4) is catalysed by 7,8-dihydroneopterin aldolase (IV). The
enzyme product is converted into dihydrofolate by the consecutive action
of 6-hydroxymethyldihydroneopterine pyrophosphokinase (V), dihydropteroate
synthase (VI), dihydrofolate synthetase (VII) and dihydrofolate reductase
(VIII). These two final steps of tetrahydrofolate (6) biosynthesis are
common chemotherapeutic targets for antibacterial and antiparasitic agents.
It could be observed that dihydropteroate synthase can be inhibited by
sulfonamides, the first synthetic antimicrobial and antiparasit drugs with
broad action spectrum. Dihydrofolate reductase can be inhibited by
trimethoprim, which acts against a variety of bacterial pathogens. However,
effective inhibitors of the early steps of folate biosynthesis could not
been found.
The characterization of the intermediates, mechanisms and enzymes of the folate pathway from plants and microorganisms (yeast, eubacteria) by molecular and structural biology, biochemistry and NMR spectroscopy is one of the major focuses of the folate group.
Biosynthesis of Tetrahydrobiopterin (BH4)
The cofactor BH4 is synthesized by only three enzymes, namely GTP Cyclohydrolase I (CYHI), 6-Pyrovoyl-H4-pterin synthase (PTPS) and Sepiapterin reductase (SR).
The biosynthetic pathway of BH4 includes minimally these three enzymes; the participation of a fourth enzyme, aldose reductase, is suggested but still controversial. However, it was shown that aldose reductase is not important for BH4 biosynthesis in liver. The structures of Escherichia coli and human GTP cyclohydrolase I, rat liver 6-pyruvoyl tetrahydropterin synthase, and mouse sepiapterin reductase have been determined by x-ray crystallography. Besides the de novo biosynthesis of BH4, SR is also known to be involved in the pterin salvage pathway catalysing the conversion of sepiapterin to dihydrobiopterin (BH2) which is transformed by dihydrofolate reductase to BH4. Furthermore,a regeneration system for the cofactor is known involving pterin-4a-carbinolamine dehydratase (PCD) and dihydropteridine reductase (DHPR). The full complement of the three BH4-biosynthesizing enzymes can be found in significant amounts in many tissues of various species. The richest sources of SR are erythrocytes, liver and brain.
Tetrahydrobiopterin (BH4) is a multifunctional cofactor for phenylalanine, tyrosine and tryptophan hydroxylases, which catalyse the initial steps in phenylalanine degradation in the liver, and are the rate-limiting steps in the biosynthesis of the neurotransmitters, catechol amines and indole amines in the brain. Tetrahydrobiopterin levels in mammalian cells are mainly determined by GTP-CH-I activity. Mutations in the GTPCH-I gene are responsible for severe diseases including dopa-responsive-dystonia and certain cases of atypical phenylketonuria. Mammalian GTP-CH-I is inhibited by tetrahydrobiopterin and stimulated by phenylalanine through complex formation with the GTP-CH-I feedback regulatory protein. A function to promote release of dopamine, serotonin and noradrenaline from the striatal and cortical nerveterminals has also been proposed for BH4. Serotonin (5-hydroxytryptamine; 5-HT) and its derivatives are neurotransmitters present in brain or pituitary gland, regulating a great number of physiological mechanisms such as sleep, appetite, thermoregulation, control of pituitary secretions and behaviour. BH4 has furthermore an essential role in the biosynthesis of nitric oxide (NO) as an allosteric activator of nitricoxide synthase (NOS) and seems to be necessary for catalytic turnover involving a redox-function of the cofactor. Recently, it was shown that an increase in BH4 biosynthesis in a pancreatic B-cell line (INS-1) is followed by enhanced NO production and subsequently, inhibition of insulin secretion. BH4 regulates human melanogenesis by forming a stable complex with the a-melanocyte stimulating hormone. Finally, BH4 is known as an essential co-factor for alkylglyceryl monooxygenases. Reduced levels of BH4 in the brain and cerebrospinal fluid are associated with several neuropsychiatric diseases such as Parkinson’s disease, Alzheimer’s disease, depression and dystonia. In atypical phenylketonuria (PKU), BH4 deficiency results in neurological disorders as a result of decreased biosynthesis of brain catecholamines and serotonin. BH4 is involved in proliferation and growth regulation of erythroid cells. Partial depletion of BH4 in a murine erythroleukaemia cell line caused inhibition of cell growth.
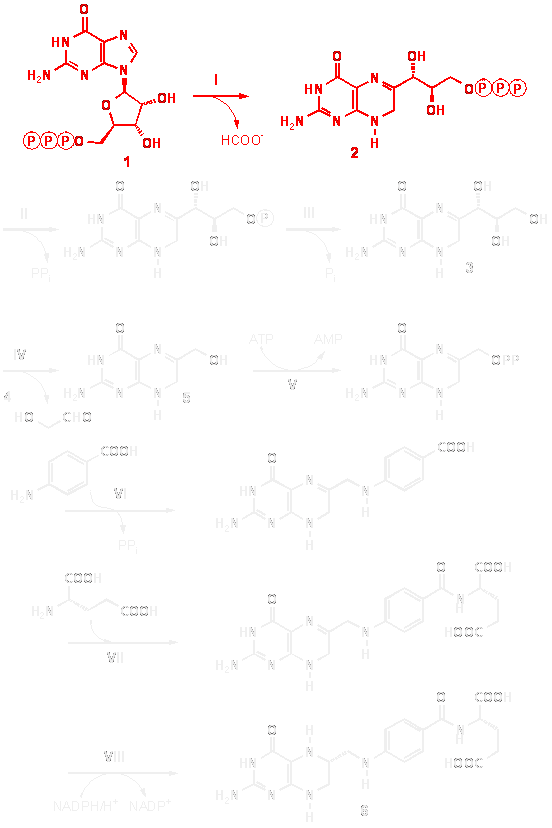
GTP cyclohydrolase I (I; E.C. 3.5.4.16) catalyzes the conversion of GTP (1) to dihydroneopterin triphosphate (2; H2NTP, 6-D-threo-1´,2´,3´-hydroxypropyl-7,8-dihydroneopterin-3´-triphosphate).
In plants and certain microorganisms, the enzyme product serves as the first committed intermediate in the biosynthesis of tetrahydrofolate.
In animals, dihydroneopterintriphosphate is converted to tetrahydrobiopterin (8; BH4, [6R]-[L-erythro-1´,2´-dihydroxypropyl]-2-amino-4-hydroxy-5,6,7,8-tetrahydropteridine) by the sequential action of 6-pyruvoyl tetrahydropterin synthase ( PTPS; E.C.4.6.1.10) and sepiapterin reductase (E.C. 1.1.1.153).